Choose the Orbital Diagram for Phosphorus.
What is the orbital diagram for phosphorus. Search only database of 12 mil and more summaries.
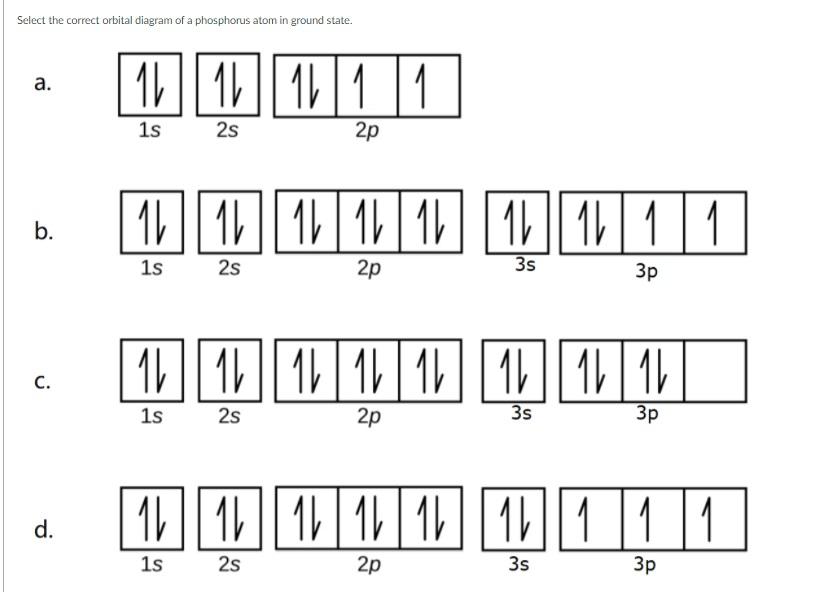
Solved Select The Correct Orbital Diagram Of A Phosphorus Chegg Com
When completing the orbital diagram for the element phosphorus which of the following statements is correct.

. 1 Get Other questions on the subject. Show all of the work needed to solve this problem. Ground state electron configuration of phosphorus is 1s 2 2s 2 2p 6 3s 2 3p 3.
In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. And then we end at three p three. Orbital diagram of Magnesium Mg 13.
Then we go to the third period and we Philip the S. How many grams of magnesium metal will react completely with 83 liters of 55 m hcl. Orbital diagram of Fluorine F 10.
P Phosphorus What element is represented by this orbital diagram. The aufbau diagram shows the. Next we go to the to P and were going to fill that up is to p six.
Science Chemistry QA Library Examine the orbital diagram for the ground state electron configuration of phosphorus. The atomic number of phosphorus is Write the electron configuration of a phosphorus atom. 11 11 Is 11 2s 101L 1L 2p 11 35 3p 11 11 11 111111 2p Is 2s 3s Зр 10 IL Is LILL 2p 11 3s 25 3p 11 Is 11 25 111111 2p 11 3s 3p Part B Determine the number of.
We have step-by-step solutions for your textbooks written by Bartleby experts. It is emphasized that the discussion about the 3d orbital participation in bonding should be based on a reasonable choice of basis sets and it seems suitable to choose the atomic orbitals in proper molecular. This number indicates the total number of electrons.
When completing the orbital diagram for the element phosphorus which of the following statements is correct. Overview of General Chemistry in Sections 13 - 16 integrated into the above learning goal for organic chemistry in sections 17 and 18. Orbital Diagram For Phosphorus.
Each sub-orbital can have a maximum of two. The p-orbital has three sub-orbitals. There are no electrons in the 3s sublevel.
What element is represented by this orbital diagram. Orbital diagram of Carbon C 7. The orbital diagram for phosphorus consists of five electrons in the third shell eight in the second and two in the first shell closest to the nucleus.
The orbital diagram for phosphorus consists of five electrons in the third shell eight in the second and two in the first shell closest to the nucleus. The bond energy for the van der waals bond between two helium atoms is 79104ev. 1s22s22p63s23p3 is the original electron configuration for PhosphorusIt will gain three electrons leaving it with the same configuration as Ar or 1s22s22p63s23p6 What.
In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. Ab initio LCAOMOSCF calculations for several typical molecules containing phosphorus have been undertaken to study the role of phosphorus 3d orbitals in the bonding.
Choose the correct orbital diagram for the ground state electron configuration of phosphorus. For the element of phosphorus you already know that the atomic number tells you the number. There is one unpaired electron in the 3p sublevel.
Mg s 2hcl aq mgcl2 aq h2 g. Advanced searches left. 57draw the orbital diagram for there are five valenceelectron in phosphorus3s3p.
Part A Choose the orbital diagram for phosphorus. Since phosphorus is in the third group in the P block elements. 2 electrons in the.
The next six electrons will go in the 2p orbital. There are electrons in the 3d sublevel. Orbital diagram for phosphorus P PhosphorusP excited state electron configuration.
Orbital diagram of Oxygen O 9. 3 points There are five electrons in the n 3 energy level. The 2p sublevel is not full.
Orbital diagram of Nitrogen N 8. Transcribed image text. There is one unpaired electron in the 3p sublevel.
Just do a little research. Collected from the entire web and summarized to include only the most important parts of it. There are no electrons in the 3s sublevel.
There are electrons in the 3d sublevel. The 2p sublevel is not full. Asked Aug 30 2019 in Chemistry by Carmenon.
First thing I would like to do for phosphorus is to ride out its electron configuration. Atoms can jump from one orbital to another by excited state. Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom.
What is the correct orbital diagram for phosphorus Study of objective drawing interpret and convert between Lewis Kekule condensed and Bond-line Structures Note. Orbital diagram of Sodium Na 12. Textbook solution for Introductory Chemistry-Selected Solution Manual 6th Edition Tro Chapter 9 Problem 5SAQ.
Orbital diagram of Aluminum Al 14. The p orbital can hold up to six electrons. So phosphorus is one s two to s to.
Orbital diagram of Phosphorus. The number of valence electrons impacts on their chemical properties and the specific ordering and properties of the orbitals are important in physics so many students have to get to grips with the basics. Assuming that the average kinetic energy of a helium atom is 32kbt at what temperature is the average kinetic energy equal to the bond energy between two helium atoms.
Can be used as content for research and analysis. Home Blog Pro Plans Scholar Login. 1 Ne 3s 3p AI Ne B I 35 3p C II.
You can obtain correct electron configurations for the elements up to. The atomic number of phosphorus is 15. Chemistry 21062019 1730 mizu36011.
The sub-orbitals are p x p y and p z. Orbital diagram of Silicon Si 15. Orbital diagram phosphorus.
V Vanadium What element is represented by this orbital diagram. This is called quantum jump. Orbital diagram of Neon Ne 11.
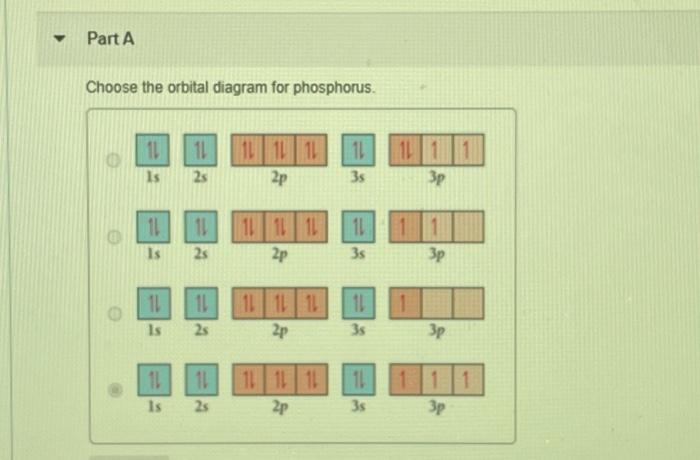
Solved Part A Choose The Orbital Diagram For Phosphorus 11 Chegg Com


0 Response to "Choose the Orbital Diagram for Phosphorus."
Post a Comment